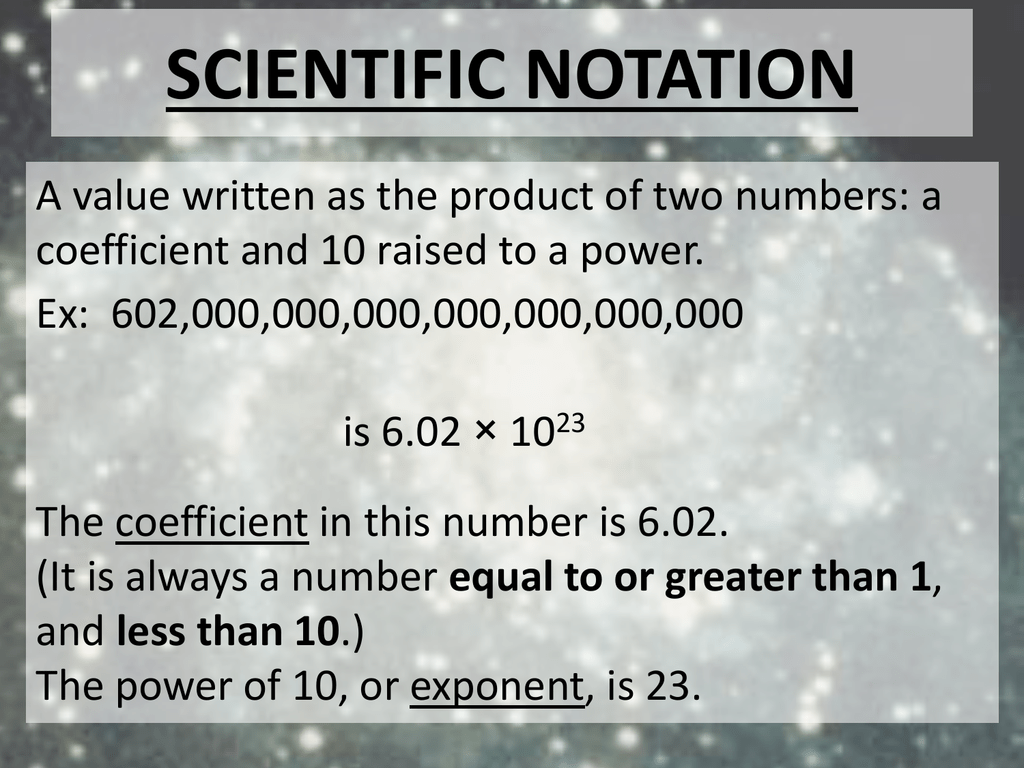
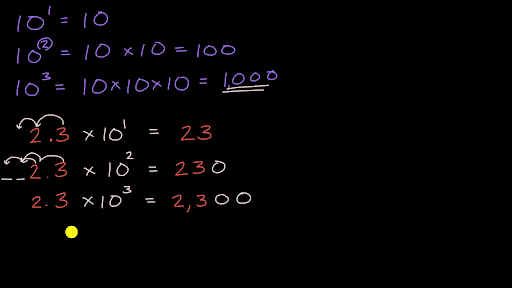What is the mass in grams of : (i) 6.022xx10^(23) atoms of oxygen ? (ii) 1.0xx10^(23) molecules ... - YouTube

hiw to solve this...pls give step by step explanation ..for your help...its answer is 1.99×10raused to - Brainly.in

You can now pair your Apple Watch with a power meter, thanks to watchOS 10 software update - BikeRadar

Auslan Tour – Goddess: Power, Glamour, Rebellion | 10 Jun & 23 Sep 2023 | ACMI: Your museum of screen culture

Calculate the number of moles for:a) 3.0115 × 10^23 atoms of copperb) 27.95 g of ironc) 1.51 × 10^23 molecules of CO2

Amazon.com: ROBERTS 10-254 23-1/2 Foot Power-Lok Carpet Stretcher Kit with 17 Locking Positions and 18 Inch Tail Block with Wheels, Including Wheeled Carrying Case , Red : Sports & Outdoors

Total kinetic energy of sample of a gas which contains 1 × 10^22 molecules is 24 × 10^2 J at 127^oC . Another sample of gas at 27^oC has a total kinetic
Perform the following calculations to the appropriate number of significant digits (6.02*10^23*4.00)÷(4.0*10^20)